Abstract
Factor IX (FIX) Padua (R338L) has been described as a game changer for hemophilia B (HB) gene therapy. The ~8-fold increased specific activity compared to wild-type FIX (FIX WT) in aPTT-based clotting assay has recently allowed for a lowering of adeno-associated virus (AAV) vector dose compared to earlier gene therapy trials using FIX WT, while still achieving sustained near-curative FIX activity levels. The lowered AAV vector dose mitigated the vector dose-dependent hepatotoxicity, which had remained a major safety and efficacy limitation for AAV gene therapy. To date, at least 2 pivotal phase III gene therapy trials for HB using AAV FIX Padua are planned. Despite this enthusiasm, the underlying molecular mechanism of the hyperactivity of FIX Padua is undefined. As such, the safety concerns of unregulated FIX activity and the potential for ensuing thrombotic complications have not been fully addressed. Indeed, recent thrombotic complications in non-gene therapy hemophilia clinical trials evaluating non-factor therapies that promote hemostasis by circumventing regulatory interactions should engender caution.
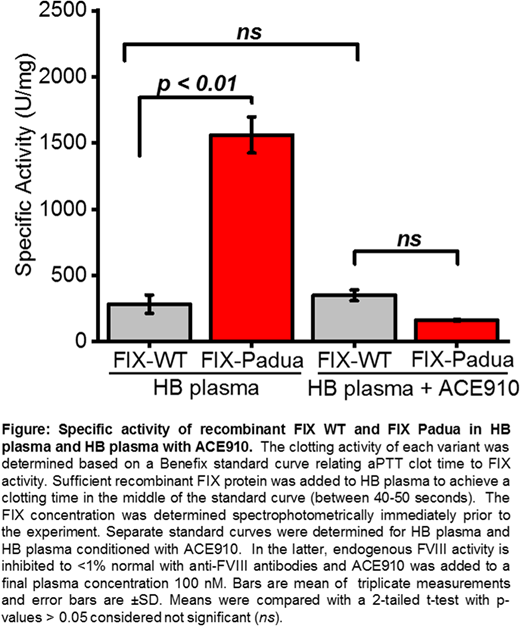
Activated FVIII (FVIIIa) is essential for the biological activity of activated FIX (FIXa), which provides an important regulatory requirement. To determine the role of FVIIIa in the hyperactivity of FIX Padua, we measured the relative specific activity of FIX Padua compared to WT in HB plasma using a unique reagent, ACE910. ACE910 is a bispecific antibody that sufficiently brings together FIXa and its substrate, FX, to efficiently promote hemostasis, even in the absence of FVIIIa molecules. It has been described as a "FVIII-mimetic" and is currently approved as a bypassing agent for hemophilia A patients with FVIII inhibitors. In our assays, plasma FVIII activity is inhibited to <1% normal activity with a cocktail of monoclonal anti-FVIII antibodies. In unadulterated HB plasma, recombinant zymogen FIX Padua has a ~8-fold higher clotting activity compared to FIX WT (Fig). However, when FVIIIa is replaced by ACE910, FIX Padua and WT have comparable clotting activities (Fig). Similarly, we observe that the hyperactivity of activated FIX Padua compared to WT in a clotting assay is completely abrogated by replacing FVIIIa with ACE910. These results suggest that the enhanced clotting activity of FIX(a) Padua compared to FIX(a) WT is wholly dependent on FVIIIa cofactor activity.
We also measured the ability of recombinant FIX(a) Padua and WT to restore thrombin production to HB plasma in a thrombin generation assay (TGA). We observe that the EC50 of thrombin generation in HB plasma of zymogen and activated FIX Padua is ~8-fold lower than for FIX(a) WT. However, when FVIIIa is replaced with ACE910, FIX(a) Padua and WT demonstrate comparable EC50s of thrombin generation. Importantly, we observe no difference in the binding of FIXa Padua and FIXa WT with ACE910 in enzyme kinetic studies; this was expected because FIXa Padua and WT have an identical ACE910 binding site in their EGF-domain. Combined, these results demonstrate that the ~8 fold hyperactivity of FIX(a) Padua compared to FIX(a) WT in plasma assays requires FVIIIa cofactor activity. Consistent with this mechanism, we have previously observed in reconstituted systems that FIX(a) Padua and WT have comparable rates of activation by FXIa and inactivation by antithrombin. This result suggests that FIX Padua and WT are regulated similarly, supporting the safety of FIX Padua as a transgene. Enzyme kinetic studies of FIXa Padua and WT without FVIIIa demonstrate similar rates of FX activation, while FIXa Padua demonstrates increased rates of FX activation with FVIIIa compared to FIXa WT.
We conclude, therefore, the mechanism of the increased specific activity of FIX(a) Padua is wholly dependent on its enhanced interaction with its cofactor FVIIIa. This enhancement is essential for the increased activity observed in both reconstituted and plasma-based assays. Moreover, the augmented interaction of FIX(a) Padua with FVIIIa is sufficient to account for the ~8-fold pro-hemostatic enhancement compared to FIX(a) WT observed in plasma assays. Combined, these results definitively address previously unanswered safety concerns regarding the potential risk of thrombotic complications with the use of FIX Padua transgene. They strongly support the ongoing use of FIX Padua in HB gene therapy.
Camire:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy; Spark Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.